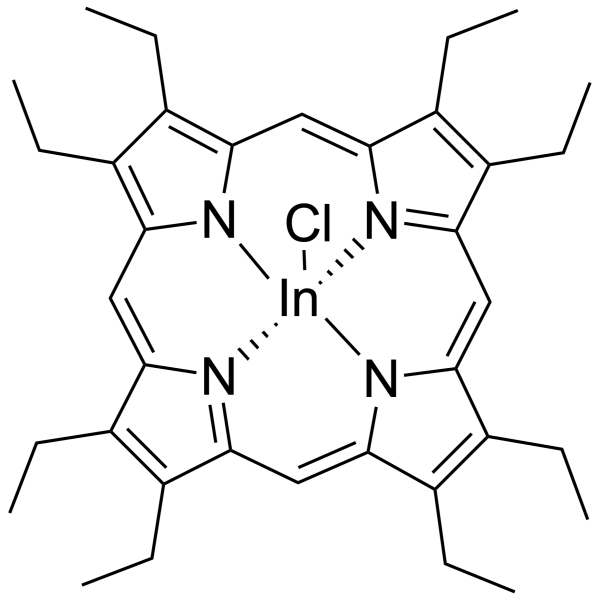
Chloride ionophore – High Purity for ISE | Selectoprobe
Price range: $416.00 through $582.00
Catalog No: SP-I-0016
Purity: 95%-98%
CAS No: 32125-07-8
Synonym: indium(III) 2-3-7-8-12-13-17-18-(octaethyl)porphyrin chloride
MW: 683.03
Formula: C36H44ClInN4
References
Zhang W, Rozniecka E, Malinowska E, et al.Optical chloride sensor based on dimer-monomer equilibrium of indium(III) octaethylporphyrin in polymeric film . Anal. Chem, 2002, 74(17): 4548-4557; DOI: 10.1021/ac0202536.
Abstract
A novel transduction chemistry for preparing optical anion-selective polymeric films that respond reversibly and selectively to chloride ion activity is demonstrated. The chloride sensors are prepared by casting thin (5-10 microm) plasticized PVC films containing indium(III) octaethylporphyrin hydroxide, along with optimized levels of a lipophilic tetraphenylborate salt, onto glass slides. When bathed in low-pH buffered solutions void of chloride, the porphyrin species spontaneously forms a hydroxide ion-bridged dimer, with the added lipophilic borate species serving as the counteranion for this complex. The maximum for the Soret absorption band of this dimeric species is shifted to 390 nm, from 410 nm for the initial monomeric porphyrin. Increases in chloride ion levels in the bathing solution results in chloride extraction and ligation to the In(III) center, and concomitant breaking of the dimer into monomeric porphyrin species, yielding a decrease in absorbance at 390 nm and an increase in optical signal at 410 nm. Under optimized conditions, optical selectivity coefficients toward chloride over a wide range of other anions (NO3-, ClO4-, SCN-, SO4(2-), F-, Br-, H2PO4-) are measured to be < 10(-3). Of all anions tested, only salicylate yields a slightly greater response than chloride. This selectivity is shown to be adequate for reversible and accurate sensing of chloride levels in diluted serum samples.
References
Qin Y, Bakker E.Elimination of dimer formation in InIIIporphyrin-based anion-selective membranes by covalent attachment of the ionophore .Anal. Chem, 2004, 76(15): 4379-4386; DOI: 10.1021/ac049577f.
Abstract
The spontaneous hydroxy-bridged dimer formation of metalloporphyrins in ion-selective membranes gives rise to a short sensor lifetime (typically days), triggered by solubility problems, the occurrence of a super-Nernstian response slope, and a pH cross response. This dimer formation is eliminated here by covalent attachment of the ionophore to the polymer matrix. Specifically, two different indium(III)porphyrins containing polymerizable groups, the chloride-selective chloro(3-[18-(3-acryloyloxypropyl)-7,12-bis(1-methoxyethyl)-3,8,13,17-tetramethylporphyrin-2-yl]propyl ester)indium(III) and the nitrite-selective Chloro(5-(4-acryloyloxyphenyl)-10,15,20-triphenylporphyrinato)indium(III), were synthesized and copolymerized with methyl methacrylate and decyl methacrylate. The covalent attachment of the ionophore to the polymer matrix indeed prevents the metalloporphyrin from forming dimeric species, as confirmed by UV/visible spectroscopy. The ion-selective membranes with grafted indium porphyrin showed Nernstian response slopes to chloride, nitrite, perchlorate, and thiocyanate anions, with a selectivity comparable to membranes with freely dissolved or underivatized metalloporphyrin. The membranes containing grafted ionophores showed a lifetime of at least two months, apparently since crystallization of the poorly soluble dimeric species may no longer occur. This is one of the first examples where the covalent attachment of an ionophore drastically improves on a number of important sensor characteristics.
| Catalog No | SP-I-0016 |
|---|---|
| Formula | C36H44ClInN4 |
| Purity | 95%-98% |
| Weight | 50mg, 100mg |
| Lead Time | 3 weeks |
| CAS No | 32125-07-8 |
| Synonym | indium(III) 2-3-7-8-12-13-17-18-(octaethyl)porphyrin chloride |
| MW | 683.03 |