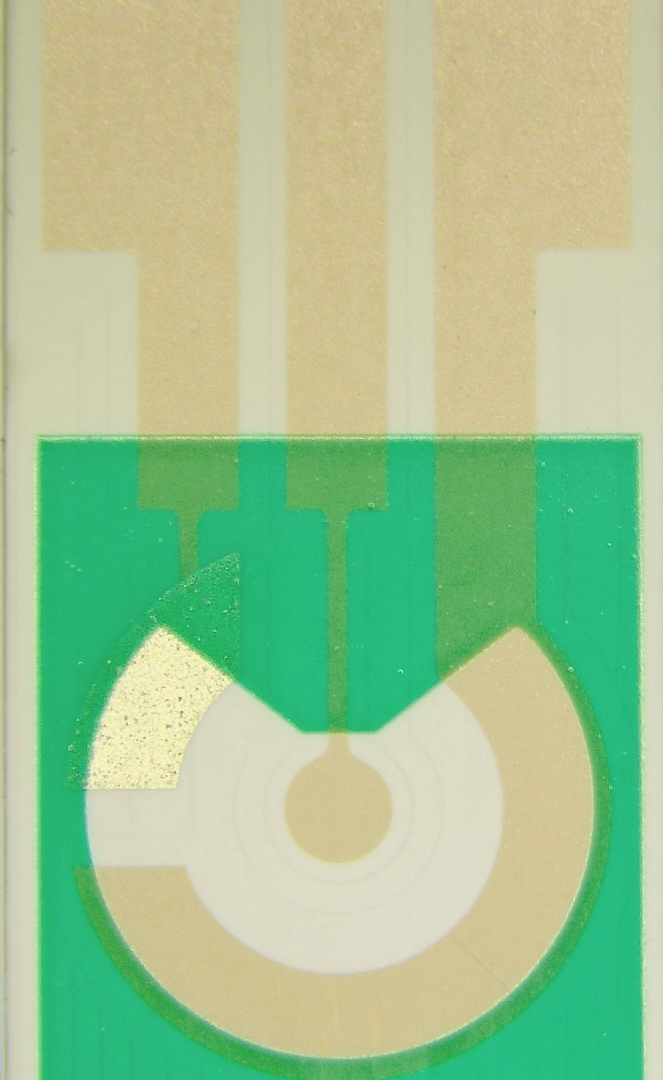
Selectoprobe Gold Screen-Printed Electrodes (SPEs)
Electrochemical Sensor
Catalog number:SP-EN-G815J
Spec.: 25 pcs/vial
Substrate size:21 x 9 mm
Working electrode material:Gold
Working electrode diameter:1.5 mm
Reference electrode material:Silver/Silver chloride
Auxiliary electrode material:Gold
Storage:Room temperature, protected from light in a dry place
Gold electrodes are among the most widely used electrochemical biosensor substrates due to their ability to be easily functionalized with thiolated biomolecules. In recent years, various alternative fabrication methods have emerged to simplify production and reduce costs. These methods include screen printing and physical fabrication using commonly available materials, making them highly suitable for biosensor applications.
Gold screen-printed electrodes (SPEs) provide a cost-effective alternative to traditional cleanroom-fabricated platforms. The screen-printing process involves depositing metal ink onto a substrate through a patterned mask, which defines the electrode’s shape. This technique is advantageous because it accommodates a wide range of substrate materials, including plastics, polymers, ceramics, and paper, while allowing for flexible electrode design.
SPEs are highly reproducible and can be mass-produced efficiently. Their surfaces can be functionalized to create immuno-, genetic-, and enzymatic sensors, highlighting their versatility for biosensor development. Additionally, by modifying the composition of the gold ink, the surface morphology and roughness of the electrodes can be tailored, further expanding their potential applications in electrochemical sensing.
References
F. V. Oberhaus, D. Frense and D. Beckmann, Immobilization techniques for aptamers on gold electrodes for the electrochemical detection of proteins: A review, Biosensors, 2020, 10(5), 45
Abstract The development of reliable biosensing platforms plays a key role in the detection of proteins in clinically and environmentally derived samples for diagnostics, as well as for process monitoring in biotechnological productions. For this purpose, the biosensor has to be stable and reproducible, and highly sensitive to detect potentially extremely low concentrations and prevent the nonspecific binding of interfering compounds. In this review, we present an overview of recently published (2017–2019) immobilization techniques for aptamers on gold electrodes for the electrochemical detection of proteins. These include the direct immobilization of thiolated aptamers and the utilization of short linkers, streptavidin/biotin interaction, as well as DNA nanostructures and reduced graphene oxide as immobilization platforms. Applied strategies for signal amplification and the prevention of biofouling are additionally discussed, as they play a crucial role in the design of biosensors. While a wide variety of amplification strategies are already available, future investigations should aim to establish suitable antifouling strategies that are compatible with electrochemical measurements. The focus of our review lies on the detailed discussion of the underlying principles and the presentation of utilized chemical protocols in order to provide the reader with promising ideas and profound knowledge of the subject, as well as an update on recent discoveries and achievements.
J. Yoon, S. N. Lee and M. K. Shin, et al., Flexible electrochemical glucose biosensor based on GOx/gold/MoS2/gold nanofilm on the polymer electrode, Biosens. Bioelectron., 2019, 140, 111343
Abstract The need for flexible biosensors has increased because of their potential applications for point-of-care diagnosis and wearable biosensors. However, flexible biosensors have low sensitivity due to the flexibility of the electrode, and their fabrication involves complex processes. To overcome these limitations, a flexible electrochemical enzyme biosensor was developed in this study by immobilizing an enzyme on the flexible polymer electrode modified with a gold/MoS2/gold nanofilm. The fabrication process involved sputter deposition of gold, spin coating of MoS2, and sputter deposition of gold on the flexible polymer electrode (commercially available Kapton® polyimide film). The flexible glucose biosensor was made by immobilization of glucose oxidase on a flexible electrode by using a chemical linker. The detection limit for glucose was estimated to be 10 nM, which indicates more sensitivity as compared with a previously reported flexible glucose sensor. This sensitivity is due to the facilitation of electron transfer by MoS2. The flexure extension of this biosensor was estimated at 3.48 mm, which is much higher than that of the rigid sensor using a gold-coated silicon electrode (0.09 mm), according to measurements with a micro-fatigue tester. The proposed flexible biosensor composed of the enzyme/gold/MoS2/gold nanofilm on the polymer electrode can be used as a flexible sensing platform for developing wearable biosensing systems because of its high sensitivity, high flexibility, and simple fabrication process.