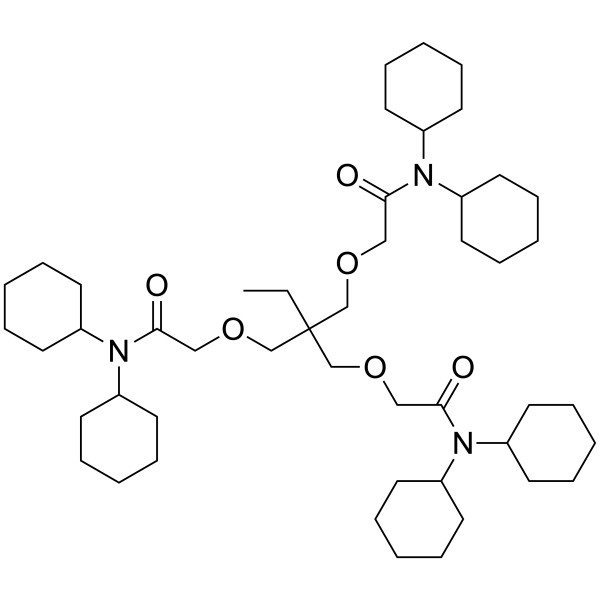
Lithium ionophore VIII – High Purity for ISE | Selectoprobe
Price range: $231.00 through $733.00
Catalog No: SP-I-0014
Purity: >97%
CAS No: 133338-85-9
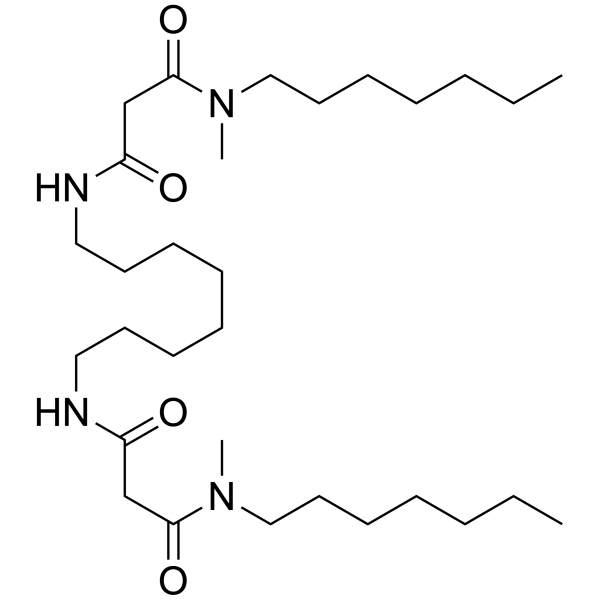
Synonym: N,N,N',N',N",N"-Hexacyclohexyl-4,4',4"-propylidynetris(3-oxabutyramide)
MW: 798.19
Formula: C48H83N3O6
References
Mikhelson K N, Bobacka J, Ivaska A, et al.Selectivity of Lithium Electrodes: Correlation with Ion−Ionophore Complex Stability Constants and with Interfacial Exchange Current Densities, 2002, 74(3):518-527; DOI: 10.1021/ac0155660.
Abstract
Lithium-selective electrodes with solvent polymeric membranes based on two different dicyclohexylamide neutral ionophores are studied systematically. The selectivity of lithium response is studied by means of the ordinary potentiometric experiments. Stability constants of lithium, sodium, and potassium ions with the neutral ionophores are measured by means of the segmented sandwich membrane method. Charge transfer through the membrane bulk and across the membrane/solution interface is studied by means of electrochemical impedance spectroscopy. Well-resolved Faradaic impedance semicircles are obtained, allowing calculation of exchange current densities for lithium, sodium, and potassium. It is clearly demonstrated that the potentiometric selectivity coefficients correlate well with thermodynamic equilibrium parameters. The correlation with exchange current densities also exists, although it is low, and seems rather qualitative than quantitative. The results are treated in favor of equilibrium at the membrane/solution interface. It is also concluded (tentatively) that the kinetic description is equivalent to the equilibrium one, giving evidence that ion−ionophore complexes form directly at the interface.
References
Albero, M. Isabel et al. Novel flow-through bulk optode for spectrophotometric determination of lithium in pharmaceuticals and saliva . Sensors and Actuators B-chemical ,2010,145 : 133-138.
Abstract
A new flow-through spectrophotometric bulk optode for the determination of lithium is reported. The optode membrane incorporates a lipophilic pH indicator (chromoionophore XIV), a lipophilic neutral ionophore (lithium ionophore VIII) and potassium tetrakis[3,5-bis(trifluoromethyl) phenyl] borate (ionic additive) as active constituents in a plasticized poly(vinyl) chloride membrane, entrapped in a cellulosic support. The composition of the membrane was tested using two different plasticizers. The optode was incorporated in a flow-injection system optimized for the determination of lithium. The analytical performance of the optode was evaluated, obtaining a linear concentration range of two decades of concentration (1 × 10−4 to 1 × 10−2 M), a limit of detection of 1.4 × 10−4 M, a fast sample throughput (25–4 samples h−1) and good reproducibility and selectivity. The sensor was seen to exhibit a fully reversible response. The proposed FI method is applied to the determination of lithium in pharmaceuticals and human saliva with satisfactory results.
Lithium ionophore VIII | C48H83N3O6 | CID 4149317 - PubChem (nih.gov)
| Catalog No | SP-I-0014 |
|---|---|
| Formula | C48H83N3O6 |
| Purity | > 97% |
| Weight | 20mg, 50mg, 100mg |
| Lead Time | 2-3 days, 3-4 weeks |
| CAS No | 133338-85-9 |
| Synonym | N,N,N',N',N",N"-Hexacyclohexyl-4,4',4"-propylidynetris(3-oxabutyramide) |
| MW | 798.19 |
You must be logged in to post a review.

Reviews
There are no reviews yet.