Calcium ionophore IV – High Purity for ISE | Selectoprobe
Price range: $336.00 through $1,304.00
Catalog No: SP-I-0008
Purity: 97%+
CAS No: 126572-74-5
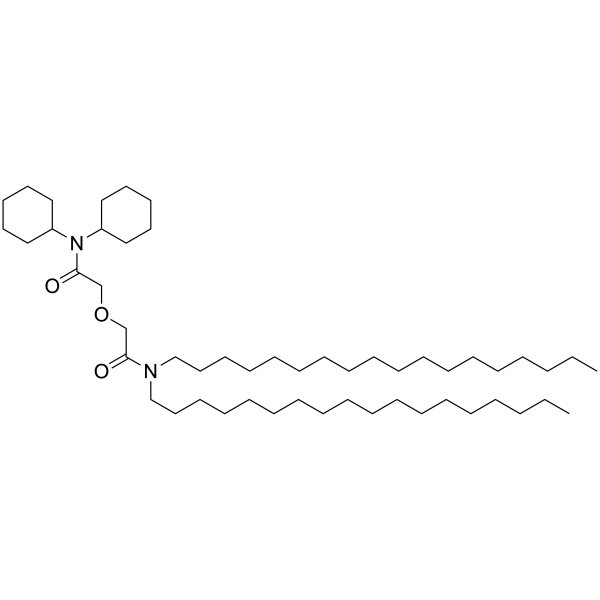
Synonyms: ETH 5234; 2-[2-(dicyclohexylamino)-2-oxoethoxy]-N,N-dioctadecylacetamide
MW: 801.36
Formula: C52H100N2O3
References
Sokalski T, Ceresa A, Fibbioli M, et al.Lowering the Detection Limit of Solvent Polymeric Ion-Selective Membrane Electrodes. 2. Influence of Composition of Sample and Internal Electrolyte Solution . Anal. Chem, 1999, 71(6):1210-1214; DOI: 10.1021/ac9809332.
Abstract
The influence of the composition of the internal electrolyte solution on the response of Pb2+- and Ca2+-selective membrane electrodes is investigated. It is shown that the lower detection limit is improved by generating, in the membrane, ionic gradients that lead to a flux of primary ions toward the inner reference electrolyte solution. If the ion flux is too strong, it may cause analyte depletion at the membrane surface and, as a consequence, apparent super-Nernstian response. Such electrodes are not adequate to measure low analyte activities but can be used to determine unbiased selectivity factors. The results are interpreted in terms of a steady-state model, introduced in the companion paper, that describes the influence of concentration gradients generated by ion-exchange and coextraction processes on both sides of the membrane.
References
Wang, Y., Wang, X., Xu, H. et al. Self-plasticized Ca2+-selective electrode with polyaniline and copolymer of aniline and 2,5-dimethoxyaniline as solid contact layers . J Mater Sci ,2024,59:16129–16140
Abstract
To address solubility, plasticizer leakage, and ionic carrier issues in PVC-based solid ion-selective electrodes, a novel plasticizer-free photocurable solid Ca2+-selective electrode (GC/PANI/CPANI/Ca2+(PBA)-ISE) was developed using a novel electrode structure. A new Ca2+-sensitive membrane based on poly(butyl acrylate) (PBA) was synthesized by ultraviolet polymerization, with conductive polyaniline (PANI) as the solid contact layer and a copolymer of aniline and 2,5-dimethoxyaniline (CPANI) as the coating. All potential electrochemical tests were carried out on the prepared Ca2+-selective electrode. The response slope of the electrode was 28.33 mV decade−1, the detection limit was 6.9 × 10−6 M, the stable linear range was 1 × 10−6 to 1 × 10−2 M, and it had a good Nernst response. The chronopotentiometric analysis and water layer tests showed that the potential drift of GC/PANI/CPANI/Ca2+(PBA)-ISE was as low as 0.0125 mV s−1 and 1.247 mV h−1. In addition, the interference tests of environmental factors were carried out. When the electrode was in different interference light and gas environments, the potential changes of 1.6 × 10−4 mV s−1 and 1.2 × 10−3 mV s−1 were observed. Compared with previous reports and commercial electrodes, the prepared GC/PANI/CPANI/Ca2+(PBA)-ISE showed good electrochemical performance, and the recovery rate in Ca2+ test solutions was higher (97.2–99.9%). This study provided theoretical insights to address solubility, plasticizer leakage, and ion carrier leakage issues in solid-state contact ion-selective electrodes. At the same time, this study provided a new structure and preparation method for the preparation of all-solid-state Ca2+-selective electrode.
Calcium ionophore IV | C52H100N2O3 | CID 601910 - PubChem (nih.gov)
| Catalog No | SP-I-0008 |
|---|---|
| Formula | C52H100N2O3 |
| Purity | 95%-98% |
| Weight | 50mg, 250mg |
| Lead Time | 2-3 days, 3 weeks |
| CAS No | 126572-74-5 |
| Synonyms | ETH 5234; 2-[2-(dicyclohexylamino)-2-oxoethoxy]-N,N-dioctadecylacetamide |
| MW | 801.36 |
You must be logged in to post a review.


Reviews
There are no reviews yet.