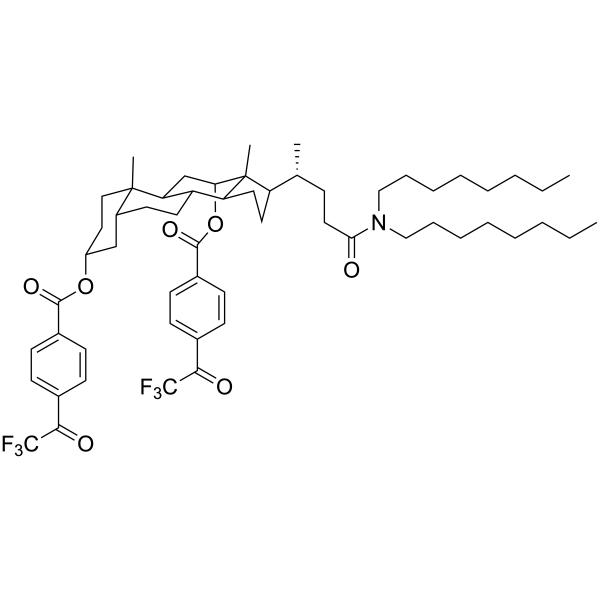
Carbonate ionophore VII – High Purity for ISE | Selectoprobe
Price range: $105.00 through $836.00
Catalog No: SP-I-0015
Purity: 97%+
CAS No: 222310-82-9
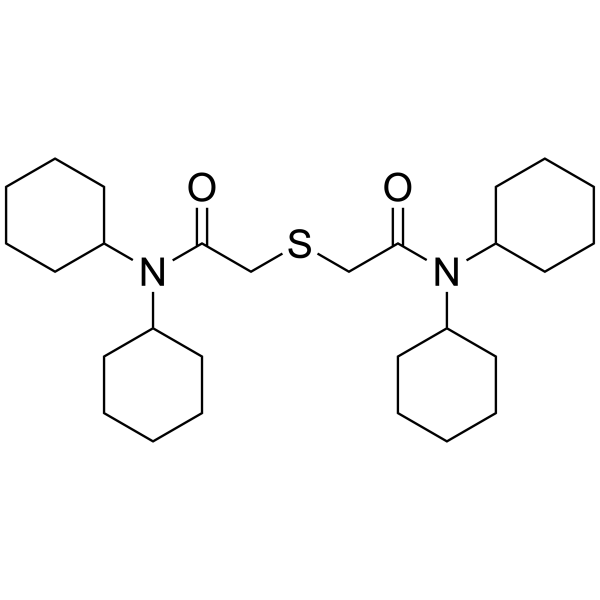
Synonym: N,N-Dioctyl-3alpha,12alpha-bis(4-trifluoroacetylbenzoyloxy)-5beta-cholan-24-amide
MW: 1016.24
Formula: C58H79F6NO7
References
Lee H J, Yoon I J, Yoo C L, et al. Potentiometric Evaluation of Solvent Polymeric Carbonate-Selective Membranes Based on Molecular Tweezer-Type Neutral Carriers , 2000, 72(19):4694-4699; DOI: 10.1021/ac991212l.
Abstract
Potentiometric properties of the ion-selective electrodes) based on highly plasticized PVC membranes doped with the carbonate-selective cholic acid (CA) derivatives have been measured. The carbonate-selective neutral carriers have been prepared by coupling one to three trifluoroacetobenzoyl (TFAB) groups to a cholic acid derivative which has three hydroxyl linkers lining on the C3, C7, and C12 positions of its rigid steroidal ring structure. The membranes based on cholic acid derivatives with two TFABs [3,7-bis(TFAB)CA, 3,12-bis(TFAB)CA, and 7,12-bis(TFAB)CA] exhibited remarkably improved carbonate selectivity, indicating that the bis(TFAB)CAs behave like molecular tweezers for the carbonate ion. For example, 3,12-bis(TFAB)CA resulted in 10−300-fold-enhanced carbonate selectivity over other anions (e.g., salicylate, ClO4-, SCN-, HPO42-, NO3-, NO2-, Br-, and Cl-) compared to that of the neutral carriers with a single TFAB group. The distances between the carbonate binding centers of bis(TFAB)CAs, i.e., the carbonyl carbons of the two TFAB groups, are in the 7.3−7.9-Å range at the AM1 level semiempirical calculation, which is too far for the carbonate ion to form direct covalent bonding. The fast atom bombardment mass spectra of bis(TFAB)CAs show that significant fractions of the compounds are either mono- or dihydrated before complexing the carbonate ion. These findings seem to suggest that bis(TFAB)CAs recognize the incoming carbonate ion by forming both covalent and hydrogen bonding between the hydrated and unhydrated TFAB groups. The analytical utility of the carbonate-selective electrode based on 3,12-bis(TFAB)CA has been demonstrated by measuring the total carbon dioxide in human serum in the presence of lipophilic anion interferents, e.g., salicylate.
References
Chikukwa MTR, Wesoly M, Korzeniowska AB, Ciosek-Skibinska P, Walker RB, Khamanga SMM. Assessment of taste masking of captopril by ion-exchange resins using electronic gustatory system. Pharm Dev Technol. 2020;25(3):281-289.
Abstract
The objective of the study was to mask the unpleasant taste of captopril (CPT). Taste masking was achieved by complexation of CPT with a basic ion exchange resin, Dowex 66, using the batch method. Dowex 66 was used for the adsorption of CPT, and physical and chemical parameters of the CPT resinates complex were evaluated. A central composite design was used to generate the experiments for the manufacture of resinates using different process and formulation variables. In vitro dissolution studies were performed for 2 h in 0.01N HCl (pH 1.6) using USP Apparatus I. The compatibility of CPT and the resin was evaluated by Fourier transform infrared (FTIR), differential scanning calorimetry (DSC), and powder X-ray diffraction (PXRD). The resinates were evaluated for micromeritic properties and further characterised using FTIR, DSC, and PXRD. Response surface methodology was used to determine the significance of input variables on the CPT content and release. The CPT resin ratio was found to have a significant impact on content of the resinates and on CPT release. The formulations were also studied for taste masking ability by means of an electronic gustatory system - electronic tongue.
Carbonate ionophore VII | C58H79F6NO7 | CID 99649076 - PubChem (nih.gov)
Documents
| Catalog No | SP-I-0015 |
|---|---|
| Formula | C58H79F6NO7 |
| Purity | 95%-98% |
| Weight | 10mg, 50mg, 100mg |
| Lead Time | 2-3 days, 3-4 weeks |
| CAS No | 222310-82-9 |
| Synonym | N,N-Dioctyl-3alpha,12alpha-bis(4-trifluoroacetylbenzoyloxy)-5beta-cholan-24-amide |
| MW | 1016.24 |